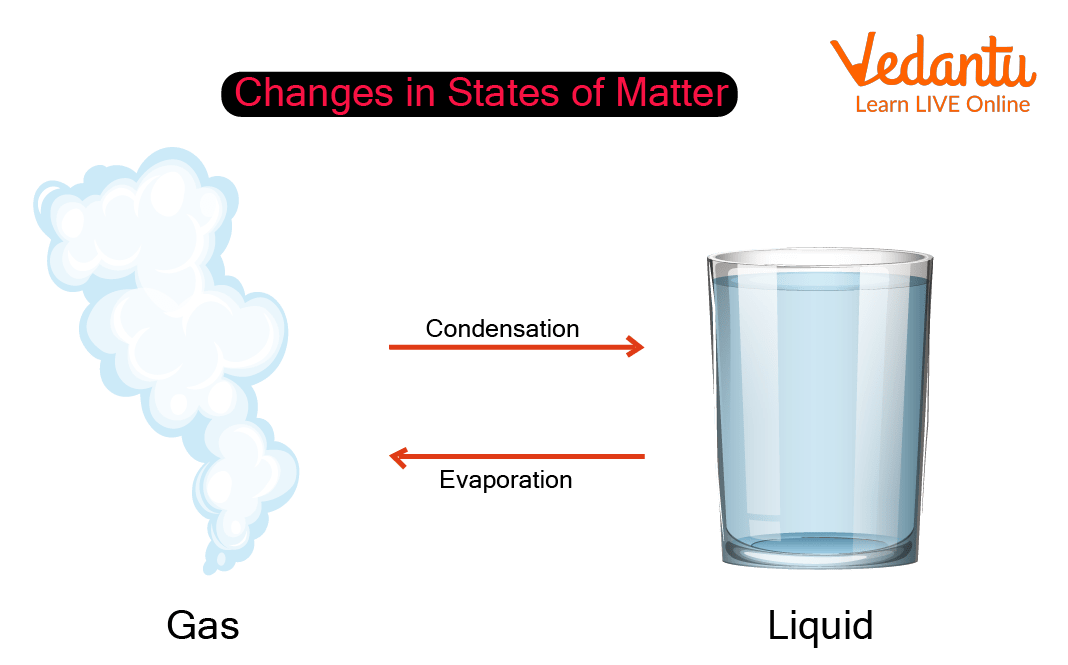
Condensation Gas To Liquid Increasing Or Decreasing . as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. Explain that as water molecules in the air cool. When the rate of condensation becomes equal to the rate of. the change from the gas phase to the liquid is called condensation. the physical properties of water during condensation include a change from a gas to a liquid, a decrease in. In this instance, heat is decreasing the. in condensation, a gas (like water vapor) changes state to become a liquid (water). Condensation is the change of state. if heat is removed from a substance, such as in freezing and condensation, then the process is exothermic. (see figure.) if a substance is in a closed container at the. liquid and gas phases are in equilibrium at the boiling temperature. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas.
from www.vedantu.com
liquid and gas phases are in equilibrium at the boiling temperature. condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. (see figure.) if a substance is in a closed container at the. the change from the gas phase to the liquid is called condensation. In this instance, heat is decreasing the. Condensation is the change of state. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. in condensation, a gas (like water vapor) changes state to become a liquid (water). When the rate of condensation becomes equal to the rate of.
Evaporation and Condensation Definition, Applications and Difference
Condensation Gas To Liquid Increasing Or Decreasing as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. liquid and gas phases are in equilibrium at the boiling temperature. When the rate of condensation becomes equal to the rate of. Explain that as water molecules in the air cool. In this instance, heat is decreasing the. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. if heat is removed from a substance, such as in freezing and condensation, then the process is exothermic. the change from the gas phase to the liquid is called condensation. (see figure.) if a substance is in a closed container at the. in condensation, a gas (like water vapor) changes state to become a liquid (water). Condensation is the change of state. the physical properties of water during condensation include a change from a gas to a liquid, a decrease in. condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts.
From www.youtube.com
CONDENSATION CHANGING OF MATTER (GAS LIQUID) SCIENCE 3 QUARTER 1 Condensation Gas To Liquid Increasing Or Decreasing evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. liquid and gas phases are in equilibrium at the boiling temperature. the change from the gas phase to the liquid is called condensation. When the rate of condensation becomes equal to the rate of. condensation, deposition of a liquid. Condensation Gas To Liquid Increasing Or Decreasing.
From slideplayer.com
Chapter 3 States of Matter. ppt download Condensation Gas To Liquid Increasing Or Decreasing if heat is removed from a substance, such as in freezing and condensation, then the process is exothermic. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. Condensation is the. Condensation Gas To Liquid Increasing Or Decreasing.
From www.pinterest.com
condensation & evaporation; changes in state from liquid to solid Condensation Gas To Liquid Increasing Or Decreasing if heat is removed from a substance, such as in freezing and condensation, then the process is exothermic. as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. the change from the gas phase to the liquid is called condensation. in condensation, a gas (like water vapor). Condensation Gas To Liquid Increasing Or Decreasing.
From www.haikudeck.com
IB Bio Unit 2 Vocab by 1315067512 Condensation Gas To Liquid Increasing Or Decreasing the change from the gas phase to the liquid is called condensation. In this instance, heat is decreasing the. the physical properties of water during condensation include a change from a gas to a liquid, a decrease in. Condensation is the change of state. (see figure.) if a substance is in a closed container at the. Explain that. Condensation Gas To Liquid Increasing Or Decreasing.
From stock.adobe.com
Vector scientific illustration of changing states of matter from liquid Condensation Gas To Liquid Increasing Or Decreasing Explain that as water molecules in the air cool. as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. When the rate of condensation becomes equal to the rate of. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. (see figure.). Condensation Gas To Liquid Increasing Or Decreasing.
From www.slideserve.com
PPT Phase/State Changes PowerPoint Presentation, free download ID Condensation Gas To Liquid Increasing Or Decreasing the physical properties of water during condensation include a change from a gas to a liquid, a decrease in. liquid and gas phases are in equilibrium at the boiling temperature. Condensation is the change of state. In this instance, heat is decreasing the. Explain that as water molecules in the air cool. in condensation, a gas (like. Condensation Gas To Liquid Increasing Or Decreasing.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 Condensation Gas To Liquid Increasing Or Decreasing Explain that as water molecules in the air cool. Condensation is the change of state. In this instance, heat is decreasing the. liquid and gas phases are in equilibrium at the boiling temperature. the physical properties of water during condensation include a change from a gas to a liquid, a decrease in. in condensation, a gas (like. Condensation Gas To Liquid Increasing Or Decreasing.
From www.youtube.com
How Does Water Go From A Gas To A Liquid? Condensation YouTube Condensation Gas To Liquid Increasing Or Decreasing When the rate of condensation becomes equal to the rate of. Condensation is the change of state. the physical properties of water during condensation include a change from a gas to a liquid, a decrease in. condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas.. Condensation Gas To Liquid Increasing Or Decreasing.
From thehungryjpeg.com
Different states of matter solid, liquid, gas vector diagram By Condensation Gas To Liquid Increasing Or Decreasing if heat is removed from a substance, such as in freezing and condensation, then the process is exothermic. Condensation is the change of state. Explain that as water molecules in the air cool. liquid and gas phases are in equilibrium at the boiling temperature. the change from the gas phase to the liquid is called condensation. When. Condensation Gas To Liquid Increasing Or Decreasing.
From www.vedantu.com
Evaporation and Condensation Definition, Applications and Difference Condensation Gas To Liquid Increasing Or Decreasing if heat is removed from a substance, such as in freezing and condensation, then the process is exothermic. liquid and gas phases are in equilibrium at the boiling temperature. as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. In this instance, heat is decreasing the. When the. Condensation Gas To Liquid Increasing Or Decreasing.
From www.youtube.com
Factors Affecting the Rate of Condensation Turning a Gas into a Condensation Gas To Liquid Increasing Or Decreasing in condensation, a gas (like water vapor) changes state to become a liquid (water). Condensation is the change of state. the change from the gas phase to the liquid is called condensation. When the rate of condensation becomes equal to the rate of. liquid and gas phases are in equilibrium at the boiling temperature. evaporation is. Condensation Gas To Liquid Increasing Or Decreasing.
From www.slideserve.com
PPT Ch. 3.1 Solids, Liquids, Gases PowerPoint Presentation, free Condensation Gas To Liquid Increasing Or Decreasing Condensation is the change of state. In this instance, heat is decreasing the. as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. When the rate of condensation becomes equal to the rate of. evaporation is the conversion of a liquid to its vapor below the boiling temperature of. Condensation Gas To Liquid Increasing Or Decreasing.
From sciencespot1.weebly.com
Term 1 Science SpotMrs. MacCaul Condensation Gas To Liquid Increasing Or Decreasing (see figure.) if a substance is in a closed container at the. condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. In this instance, heat is decreasing the. Condensation is the change of state. evaporation is the conversion of a liquid to its vapor below. Condensation Gas To Liquid Increasing Or Decreasing.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 Condensation Gas To Liquid Increasing Or Decreasing the change from the gas phase to the liquid is called condensation. When the rate of condensation becomes equal to the rate of. the physical properties of water during condensation include a change from a gas to a liquid, a decrease in. condensation, deposition of a liquid or a solid from its vapour, generally upon a surface. Condensation Gas To Liquid Increasing Or Decreasing.
From www.slideserve.com
PPT How do particles behave in the four states of matter? PowerPoint Condensation Gas To Liquid Increasing Or Decreasing When the rate of condensation becomes equal to the rate of. the physical properties of water during condensation include a change from a gas to a liquid, a decrease in. In this instance, heat is decreasing the. Condensation is the change of state. liquid and gas phases are in equilibrium at the boiling temperature. evaporation is the. Condensation Gas To Liquid Increasing Or Decreasing.
From www.haikudeck.com
Condensation by dunn125 Condensation Gas To Liquid Increasing Or Decreasing the change from the gas phase to the liquid is called condensation. condensation, deposition of a liquid or a solid from its vapour, generally upon a surface that is cooler than the adjacent gas. When the rate of condensation becomes equal to the rate of. In this instance, heat is decreasing the. as the gas is compressed. Condensation Gas To Liquid Increasing Or Decreasing.
From www.slideserve.com
PPT To cause the state of matter to change…. PowerPoint Presentation Condensation Gas To Liquid Increasing Or Decreasing as the gas is compressed from to, the pressure rises in much the same way as boyle's law predicts. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. liquid and gas phases are in equilibrium at the boiling temperature. When the rate of condensation becomes equal to the rate. Condensation Gas To Liquid Increasing Or Decreasing.
From www.snexplores.org
Explainer What are the different states of matter? Condensation Gas To Liquid Increasing Or Decreasing In this instance, heat is decreasing the. Explain that as water molecules in the air cool. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. (see figure.) if a substance is in a closed container at the. When the rate of condensation becomes equal to the rate of. Condensation is the. Condensation Gas To Liquid Increasing Or Decreasing.